It is also the only vaccine with proven efficacy against 3 variants. To prevent RSV LRTI related illness in.

The Respiratory Syncytial Virus Vaccine Landscape Lessons From The Graveyard And Promising Candidates The Lancet Infectious Diseases
ResVax - RSV F Vaccine Infants via Maternal Immunization Respiratory Syncytial Virus RSV RSV F Vaccine Older Adults 60 Years Respiratory Syncytial Virus RSV.
Novavax rsv vaccine. NVAX a late stage biotechnology company developing next-generation vaccines for serious infectious diseases today. Novavax RSV vaccine for older adults gets fast-tracked Respiratory Syncytial Virus--Courtesy of NIAID Novavax NVAX is racing to get its vaccine against respiratory syncytial virus. Novavax is funding the RSV program with the help of 89 million from the Bill and Melinda Gates Foundation.
To provide protection against RSV LRTI for young infants via maternal immunization Pediatrics Phase 1. NIST Clarifies Structure of Prospective Vaccine for Respiratory Virus This figure shows one possible structure of the nanoparticles about 30 nanometers from end to end roughly 15 times a strand of DNAs width analyzed in the study. During the first 90 days of life the percentage of infants with RSV-associated medically significant lower respiratory tract infection was 15 in the vaccine group and 24 in the placebo group.
Overview of RSV F Vaccine Pipeline Novavax is focused on 3 target populations. Maternal immunization MI pediatrics and the elderly MI Phase 2. Novavax clinical trial participants who did not receive the full 2-dose series of the active COVID-19 vaccine candidate should be counseled by trial investigators to follow current prevention measures to protect themselves against COVID-19 and offered an FDA-authorized COVID-19 vaccine series PREVENT-19 Novavax study to assess the safety.
The company highlighted progress in its various vaccine programs including the ongoing Phase 3. 30 the company had spent 119 million on RD most of which stemmed from activities related to its RSV vaccine. The Novavax COVID-19 vaccine codenamed NVX-CoV2373 is a subunit COVID-19 vaccine candidate developed by Novavax and the Coalition for Epidemic Preparedness Innovations CEPI that is undergoing trials in India under the brand name Covovax.
Novavax RSV Vaccine Fails to Hit Primary Endpoint in Phase III Trial Published. Has suffered yet another major setback in its quest to get its respiratory syncytial virus vaccine to market. We are very encouraged that the Novavax maternal RSV vaccine reduced severe RSV hypoxemia by 60 in the first months of life and believe this vaccine has great potential for reducing.
GAITHERSBURG Md Sept. Feb 28 2019 By Alex Keown Shares of Maryland-based Novavax are down 66 percent in morning trading after the company announced its respiratory syncytial virus RSV vaccine called ResVax failed to hit its primary endpoint in a Phase III trial. Novavax is in addition to the adult studies also running mid-stage trials of its RSV F Vaccine to protect infants via maternal immunization.
European regulators arent on board. 28 2020 GLOBE NEWSWIRE -- Novavax Inc. It requires two doses and is stable at 2 to 8 C 36 to 46 F refrigerated temperatures.
Reuters - Novavax Inc said data from a mid-stage study showed that immunizing pregnant women with its vaccine for a common respiratory virus. Novavax reported its Q4 earnings on March 14. Novavax has reported negative top-line results from the Phase III Prepare clinical trial of its ResVax vaccine being developed to prevent respiratory syncytial virus RSV disease in infants through maternal immunisation.
The Novavax Covid vaccine has the highest efficacy against the original Covid-19 strain and the South Africa variant. Novavax is making news these days mostly for its COVID-19 vaccine work but its been working for years to advance its RSV and flu programs.
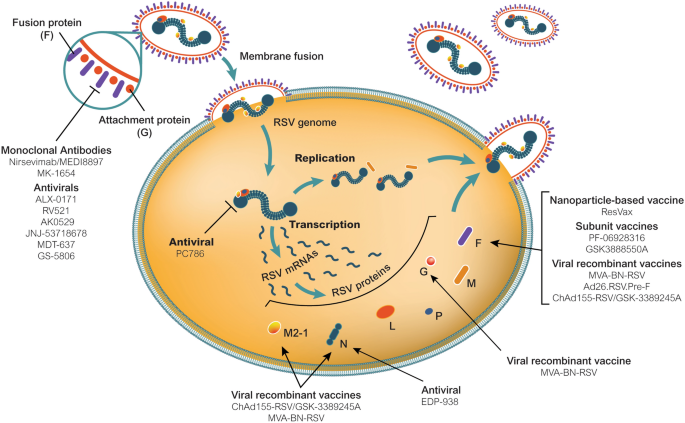
The Future Of Respiratory Syncytial Virus Disease Prevention And Treatment Springerlink
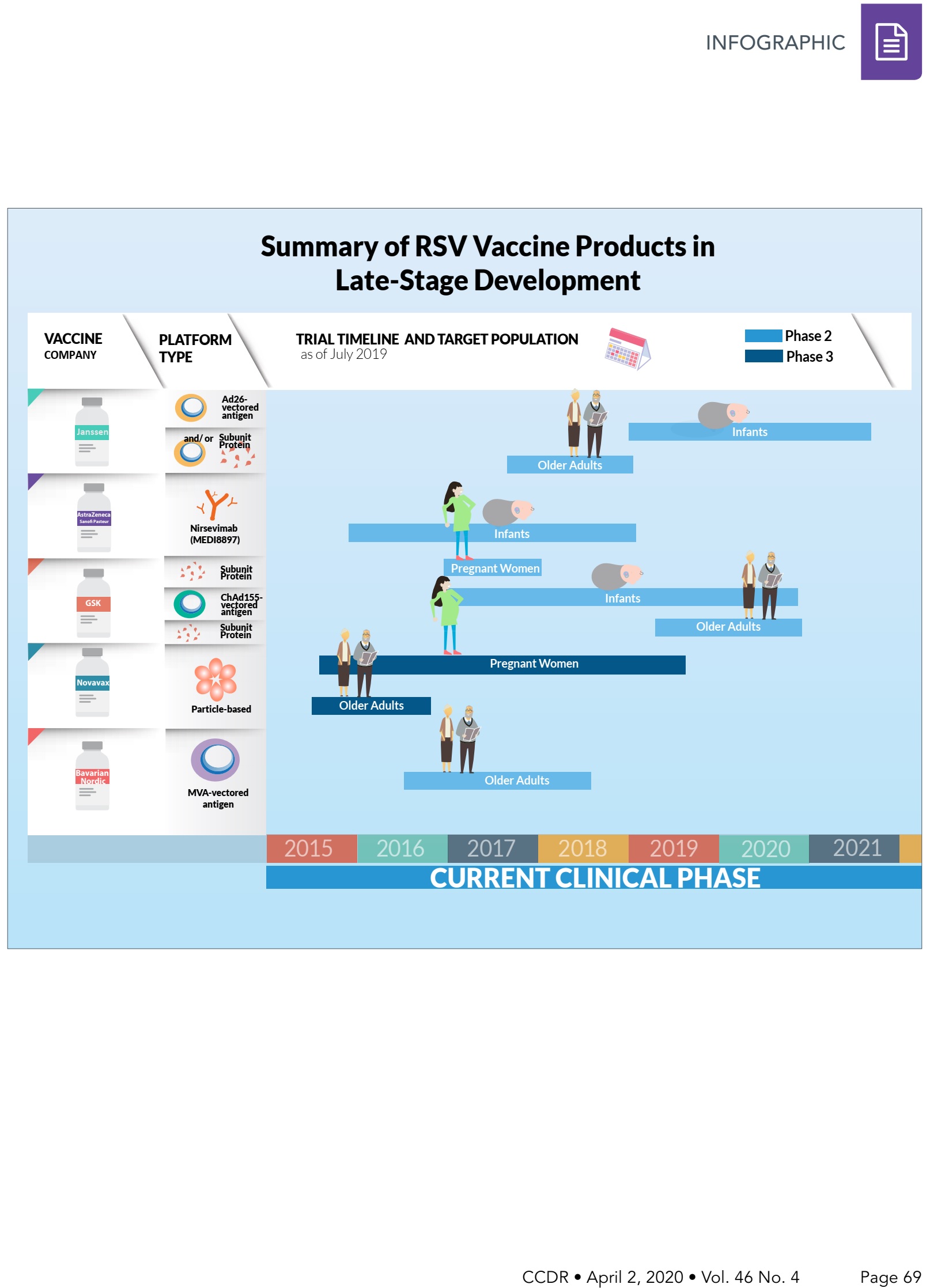
Summary Of Rsv Vaccine Products In Late Stage Development Infographic Ccdr 46 4 Canada Ca

Coronavirus Sars Cov 2 Covid 19 Vaccine Research Molecular Devices

Unsuccessful Phase Iii Could Determine Novavax Rsv Vaccine Failure
Comments